Who is Eligible
In hospice or hospice eligible – Is currently receiving or qualifies for hospice care at home or at a hospice care facility.
Agitation – Shows symptoms of agitation. This would be determined by an assessment done by study staff members at a study site.
Dementia Diagnosis – Has Alzheimer’s disease or any type of dementia.
Adults, age 40 or older.
Study Partner – A paid or unpaid caregiver who has frequent contact with the study participant and agrees to take part in all in-person and telephone visits.
Legally Authorized Representative (LAR) – A person authorized to make medical decisions on behalf of the participant if the participant is not able to make medical decisions on their own.
What happens before joining the LiBBY Study?
Before joining the study, interested caregivers and potential participants will contact the nearest study site location to talk about the study in more detail.
Those interested will receive additional information about study visit procedures, the study medication and possible side effects, and tests to evaluate potential participants’ symptoms.
After this discussion, potential participants or their caregivers will decide if they would like to undergo screening for the study to confirm eligibility.
What will participation in the study involve?
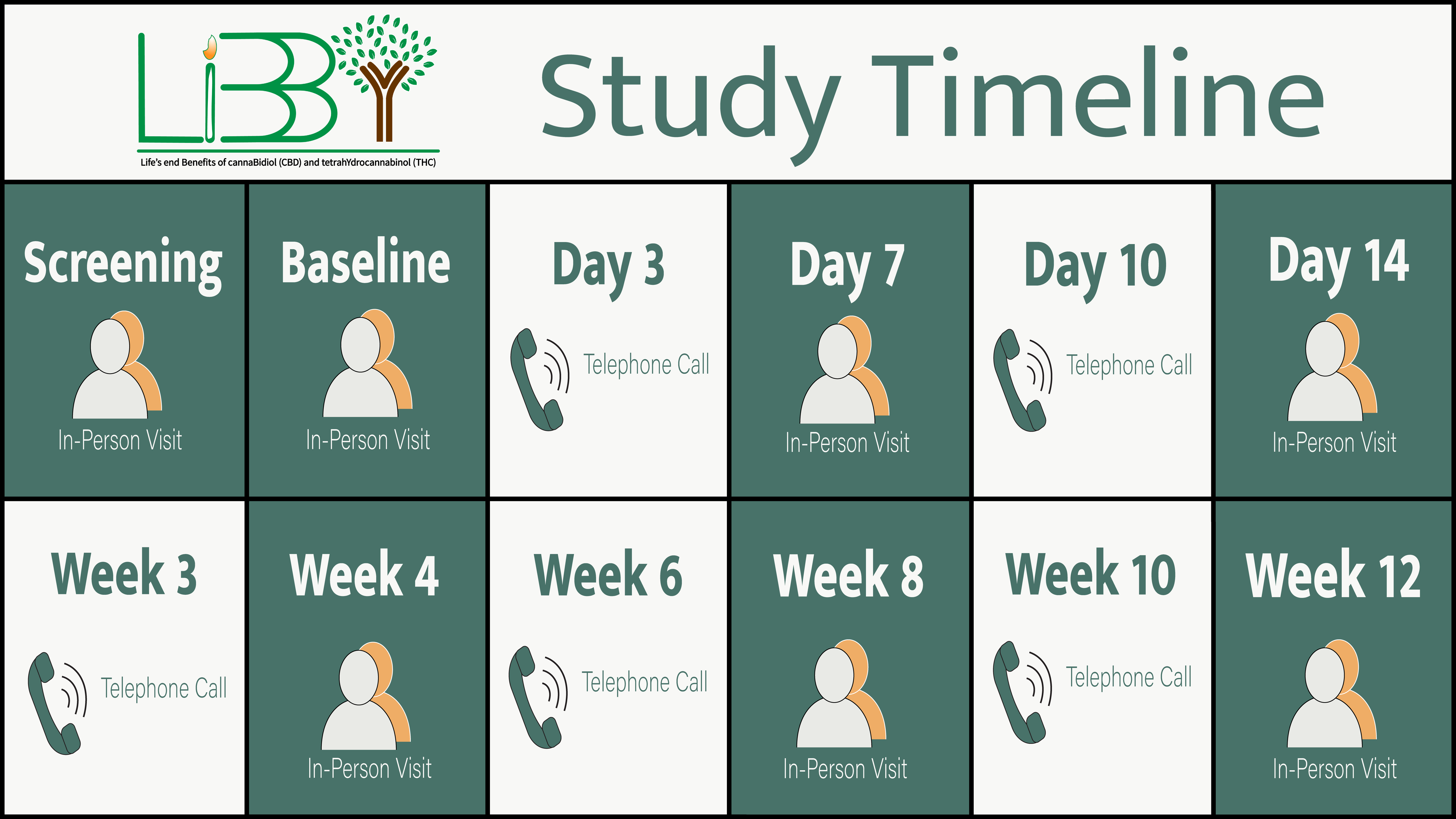

Agreement to participate for 12 weeks, involving in-person and telephone visits with study researchers.
It is understood that end-of-life stages can be unpredictable. The participant’s involvement in the study may be adjusted to fit individual circumstances.

Participation of a study partner.
A study partner is someone who has contact with the study participant at least 5 hours per week and is able to participate in all in -person and telephone visits.

Agreement to take the study medication as instructed by study staff.

Agreement not to use cannabinoids (THC and CBD) in any form during the study, other than the study medication that will be given by study staff.
All participants are required to have a study partner that:

Is a loved one and/or caregiver who has contact with the participant at least 5 hours per week.

May be a person that helps with the study participant’s care, for example a hospice care nurse or home health aide.

Is able to provide accurate information about the participant to the study team.

Will play an important role in helping researchers track changes in the participant’s behavior and symptoms.
Legally Authorized Representative
It is understood that in most cases the study participant will depend on a caregiver or loved one(s) to support them in their final stages of life. The LiBBY Study requires a study partner and may require a Legally Authorized Representative (LAR).
It’s possible the same person may be the participant’s study partner and LAR.
